The chemistry of life happens so fast that a millionth of a second is an eternity—an eternity that is largely invisible to science. In that time, molecules change in ways we cannot see. Now, though, there is a way of looking at molecules in solution—as they are in life—that shows both the big-picture shape and smaller-scale details of a molecule in one glance, with each glimpse being captured in one hundred trillionths of a second. With this technique, new chemical landscapes are now open to study.
Working at the BioCARS beamline 14-ID at the U.S. Department of Energy’s Advanced Photon Source at Argonne National Laboratory, researchers from the National Institutes of Health and The University of Chicago have combined simultaneous small-angle and wide-angle x-ray scattering (SAXS and WAXS) with an ultrafast laser to observe the dynamics of a protein in solution with 100-picosecond time resolution and 2.5-Å spatial resolution. The team demonstrated this unprecedented capability in a study of myoglobin.
Myoglobin is the protein that stores oxygen in muscles. The researchers looked at a reversible transition in which a carbon monoxide (CO) molecule binds to and releases from the active site in myoglobin. (The CO molecule is a stand-in for an oxygen molecule: They are chemically similar but the CO reaction is easier to observe with this technique.) The structures with and without CO are known, but are nearly identical. How, then, does the molecule change as CO gets out?
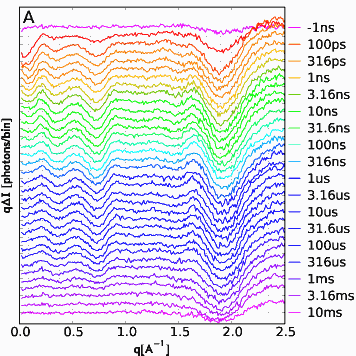
In the experiment, the release of CO was triggered by a pulse of laser light. At a given time delay ranging from 100 picoseconds to 10 milliseconds after the laser pulse, the sample was exposed to an x-ray pulse (see figure). X-rays that scatter at small angles (SAXS), tell about the overall shape of the myoglobin molecule. X-rays scattered at wide angles away from the sample (WAXS) give more detailed information about the structure.
The SAXS data show that the molecule immediately expands, but after 10 nanoseconds have passed, it’s back to nearly normal size. In addition, signatures in the scattering data are consistent with the presence of structures different from the original, and these signatures evolve over time. And because of the high spatial resolution of this technique, it may also be able to track when the CO molecule moves within the myoglobin structure and when it is excreted from the protein.
The method is made possible by a unique laser “pump-probe” setup developed in collaboration with NIH scientists as part of recent upgrade to beamline 14-ID, which is operated by BioCARS, part of the University of Chicago Center for Advanced Radiation Sources. The setup features a high-power, broadly tunable, short pulse laser system combined with sophisticated capabilities for timing the arrival of the laser pulse in relation to the x-ray pulse. In addition, given the design of the scattering diffractometer, the x-ray detector is large enough to capture both small- and wide-angle scattering without the need to reposition the detector.
Because this technique allows researchers to study molecules in solution rather than in single crystals, to collect SAXS and WAXS data simultaneously, to probe at the picosecond time scale, and to use light as a trigger, it is now possible to ask entirely new questions about a wide range of chemical and biological systems. — Jane Andrew
See: Hyun Sun Cho1, Naranbaatar Dashdorj1, Friedrich Schotte1, Timothy Graber2, Robert Henning2, and Philip Anfinrud1*, “Protein Structural Dynamics in Solution Unveiled via 100-ps Time-resolved X-ray Scattering,” PNAS, 107 (16), 7281 (20 April 2010). DOI: 10.1073/pnas.1002951107
Author affiliations: 1National Institutes of Health, 2The University of Chicago
Correspondence: *[email protected].
Use of BioCARS Sector 14 was supported by the National Institutes of Health (NIH), National Center for Research Resources, under Grant RR007707. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science, Office of Basic Energy Sciences (DOE-BES). The APS is the source of the Western Hemisphere’s brightest high-energy x-ray beams for research in virtually every scientific discipline. More than 3,500 scientists representing universities, industry, and academic institutions from every U.S. state and several foreign nations visit the APS each year to carry out applied and basic research in support of the BES mission to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels in order to provide the foundations for new energy technologies and to support DOE missions in energy, environment, and national security. To learn more about the Office of Basic Energy Sciences and its x-ray user facilities. Use of the APS was supported by the DOE-BES, under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.