Many patients suffering from respiratory problems such as asthma experience quick and effective relief by inhaling medication present in a mist or spray. The most popular type of device for delivering a measured amount of aerosolized medication is the metered-dose inhaler (MDI). Although MDIs are compact, inexpensive, and generally effective for delivering inhaled medications, many patients receive less than the optimum drug dose. In order to improve device performance, optical imaging and other techniques have been used to characterize how the spray is distributed. However, these traditional techniques provide only limited information about medication dispersion within the quickly-evolving spray plume, and cannot directly visualize the concentration of the drug. Now these limitations have been overcome by subjecting MDI sprays to x-ray fluorescence spectroscopy (XFS), carried out at X-ray Science Division beamline 7-BM-B of the U.S. Department of Energy’s Advanced Photon Source (APS), an Office of Science user facility at Argonne National Laboratory. In a series of experiments, changing the distribution of bromine (an element found in some common inhaler drugs) within MDI sprays was determined with millisecond precision. The team behind this research expects their experimental findings will lead to improvements in the drug-delivery performance of metered-dose inhalers.
Drug delivery from MDIs to the lungs can vary widely, from 40% to 10% of the medication present in each spray. Two factors are chiefly responsible for patients receiving low drug doses: incorrect operation of the device, and less-than-optimum performance of the MDI device itself. Device performance is directly related to how the drug is distributed within the spray; for instance, drug concentration may be higher in one region of the spray plume than another, potentially impeding drug delivery to the lungs. Understanding how the medication is precipitated, concentrated, and distributed within MDI sprays is therefore essential to improving their performance.
Many techniques have been employed to characterize MDI sprays. High-speed optical imaging methods, e.g., Schlieren photography and shadowgraphs, measure the expansion of the spray plume and its rapidly-changing density variations. However, these methods cannot differentiate the medication from the spray's propellant. Although other diagnostic tools (e.g., particle filtration, various laser techniques) can determine medication distribution within the spray plume, their effectiveness is limited to areas far downstream of the nozzle where the spray becomes diluted. In contrast, the dynamic phenomena of most interest, such as precipitation of medication from the vapor plume, occur largely in the “near-field” region extending from just inside the nozzle out to the first few millimeters.
Fortunately, synchrotron-based XFS is well suited for probing the near-field spray region. By causing the drug to fluoresce, its distribution and concentration throughout the evolving spray plume can be mapped with very high spatial (5 µm) and time (1 ms) resolutions. Moreover, XFS can measure the drug's concentration within the multitude of droplets that suddenly appear and grow throughout the spray.
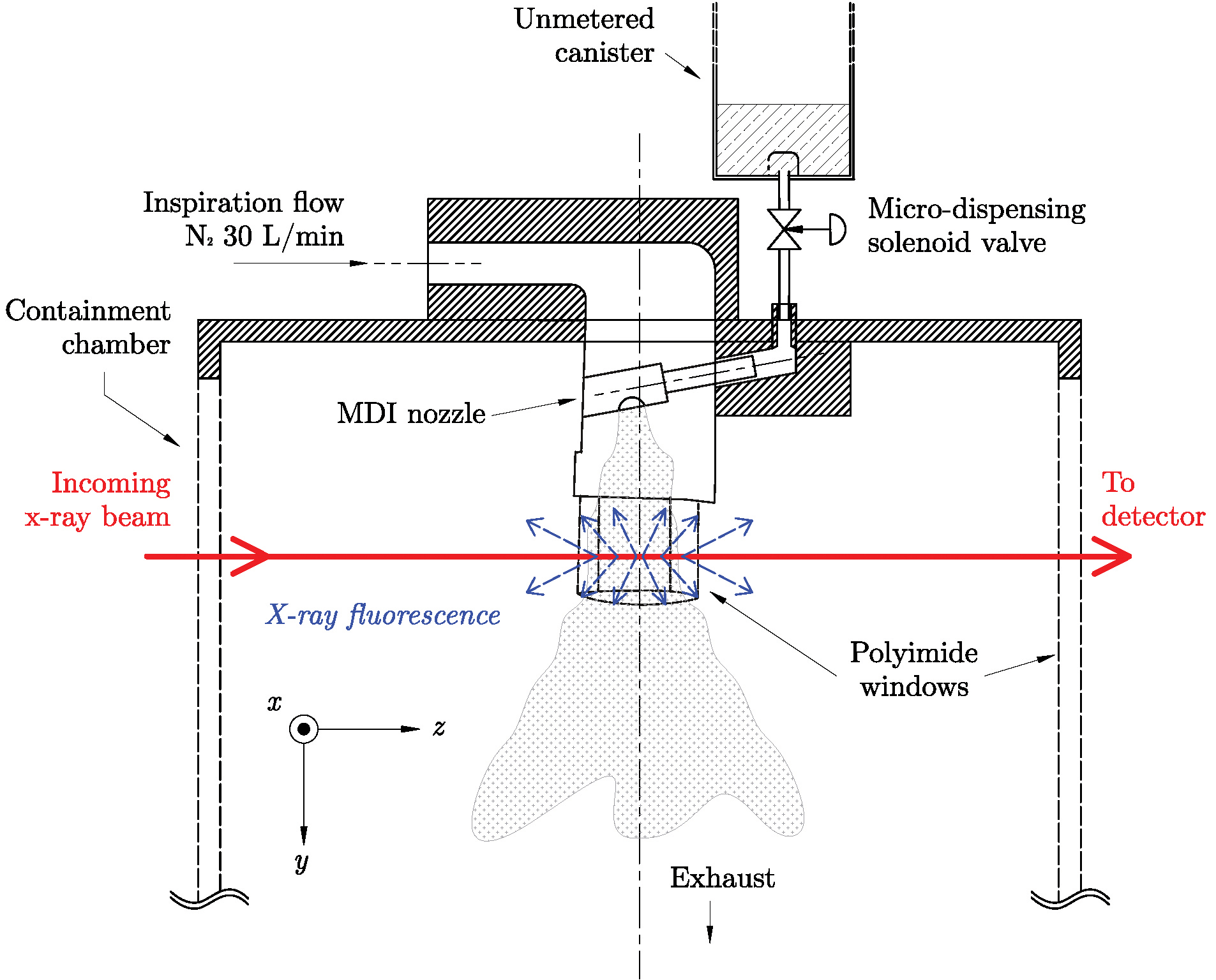
Figure 1 depicts the basic experimental layout at 7-BM-B utilized by researchers from Argonne, Monash University (Australia), the University of Sydney (Australia), and Chiesi Limited (UK). The spray apparatus was constructed to mimic the spray from a real-world inhaler, including incorporating an actual MDI nozzle. The pressurized spray was composed of ipratropium bromide (the active drug) dissolved in ethanol and mixed with a standard hydrofluoroalkane (HFA) propellant. At spray initiation, focused synchrotron x-rays (the red line in Fig. 1) ejected the innermost (K level) electrons within the bromine atoms. Higher-level (L and M) electrons that replaced the missing core electrons caused the bromine atoms to emit characteristic x-rays. By measuring the emitted x-ray fluorescence, bromine distribution and concentration were determined. The energy of the x-rays selected for the experiment (15 keV) is capable of exciting bromine, while the emitted fluorescence is only weakly absorbed by the spray's ethanol and propellant components.
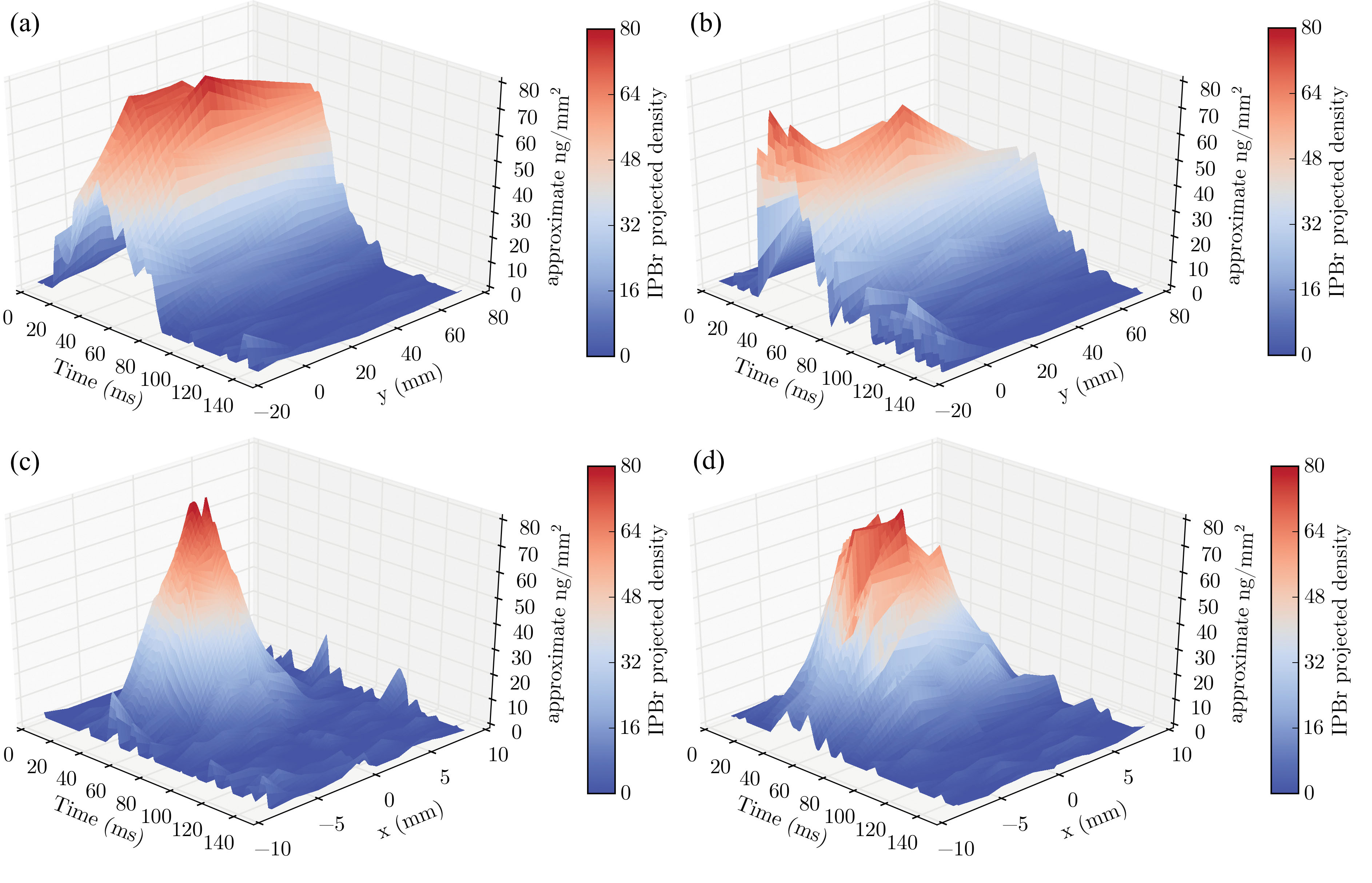
Bromine concentration and distribution is depicted in the three-axis graphs of Fig. 2. Panels 2(a) and (b) show how bromine concentrations changed with distance using two different propellants (HFA-134a and HFA-227). Panels 2 (c) and (d) reveal that drug concentration was highest around the centerline of the mouthpiece.
While bromine appears in many common inhaler medications, other inhaler drugs contain elements that also fluoresce for properly-tuned x-rays. Besides examining additional inhalant drugs, future experiments will measure how variations in nozzle design affect drug profiles in the critical near-field spray region. Moreover, combining XFS with laser excitation will aid in the measurement of drug precipitation and vaporization rates.
The ultimate aim of these experiments is improving MDI design to increase the fraction of medication consistently delivered to the lungs. — Philip Koth
See: Daniel J. Duke1*, Alan L. Kastengren1, Nicholas Mason-Smith2, Yang Chen3, Paul M. Young3, Daniela Traini3, David Lewis4, Daniel Edgington-Mitchell2, and Damon Honnery3, “Temporally and Spatially Resolved x-ray Fluorescence Measurements of in-situ Drug Concentration in Metered-Dose Inhaler Sprays,” Pharm. Res. 33, 816 (2016). DOI 10.1007/s11095-015-1828-6
Author affiliations: 1Argonne National Laboratory, 2Monash University, 3University of Sydney, 4Chiesi Limited
Correspondence: *[email protected]
This research was supported by the Australian Research Council. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
Inhaler photo: wikimedia.org/wikipedia/commons/e/e4/Metered-dose_Inhaler.JPG
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.