The original DESY press release can be seen here.
Using three synchrotron x-ray light sources, including the U.S. Department of Energy’s Advanced Photon Source (APS), an Office of Science user facility at Argonne, scientists have discovered two new iron oxides. The discovery points to a huge, hitherto unknown oxygen source in the lower mantle of the Earth. The results were reported in Nature Communications.
Iron oxides in nature take on different forms. “The most common iron oxide is hematite, Fe2O3, which is the end product of many geological processes and the main source of iron for our civilization,” said Elena Bykova (University of Bayreuth), the lead author of this new study. During the past five years, however, scientists have discovered other iron oxides like Fe4O5, Fe5O6, and Fe13O19 that form at high pressures and temperatures. In order to further investigate the behavior of hematite and magnetite (Fe3O4), Bykova and her colleagues from the University of Bayreuth (Germany), the Deutsches Elektronen-Synchrotron (DESY, Germany), The University of Chicago, and the European Synchrotron Radiation Facility (ESRF, France) used x-ray diffraction on samples in laser-heated diamond anvil cells at the GeoSoilEnviroCARS (GSECARS) x-ray beamline 13-ID-D at the APS, ESRF beamline ID09A, and beamline P02.2 at PETRA III (DESY), as well as Mössbauer spectroscopy on the ESRF ID18 beamline. This allowed a detailed characterization of structural, chemical, and electronic processes in the iron oxide system under extreme conditions.
“In this so-called diamond anvil cell, a minute sample can be compressed between two diamonds to several hundred thousand times the atmospheric pressure while a meticulously aligned laser can also heat the sample through the transparent diamond anvils to several thousand degrees Celsius,” said DESY scientist Hanns-Peter Liermann, a co-author of the paper. At the same time, the exceptionally bright and small x-ray beams from the synchrotron light sources can track structural changes in the sample.
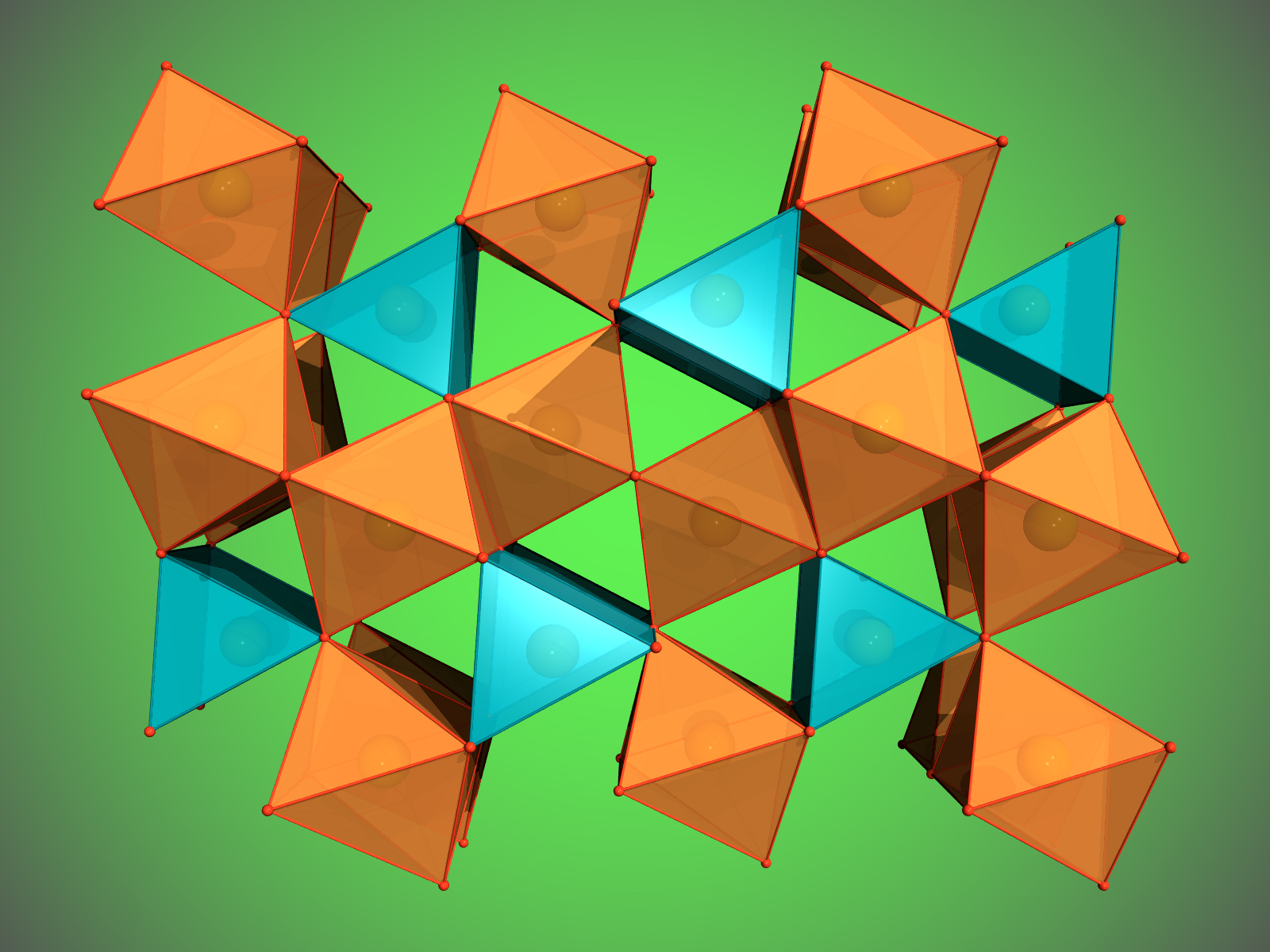
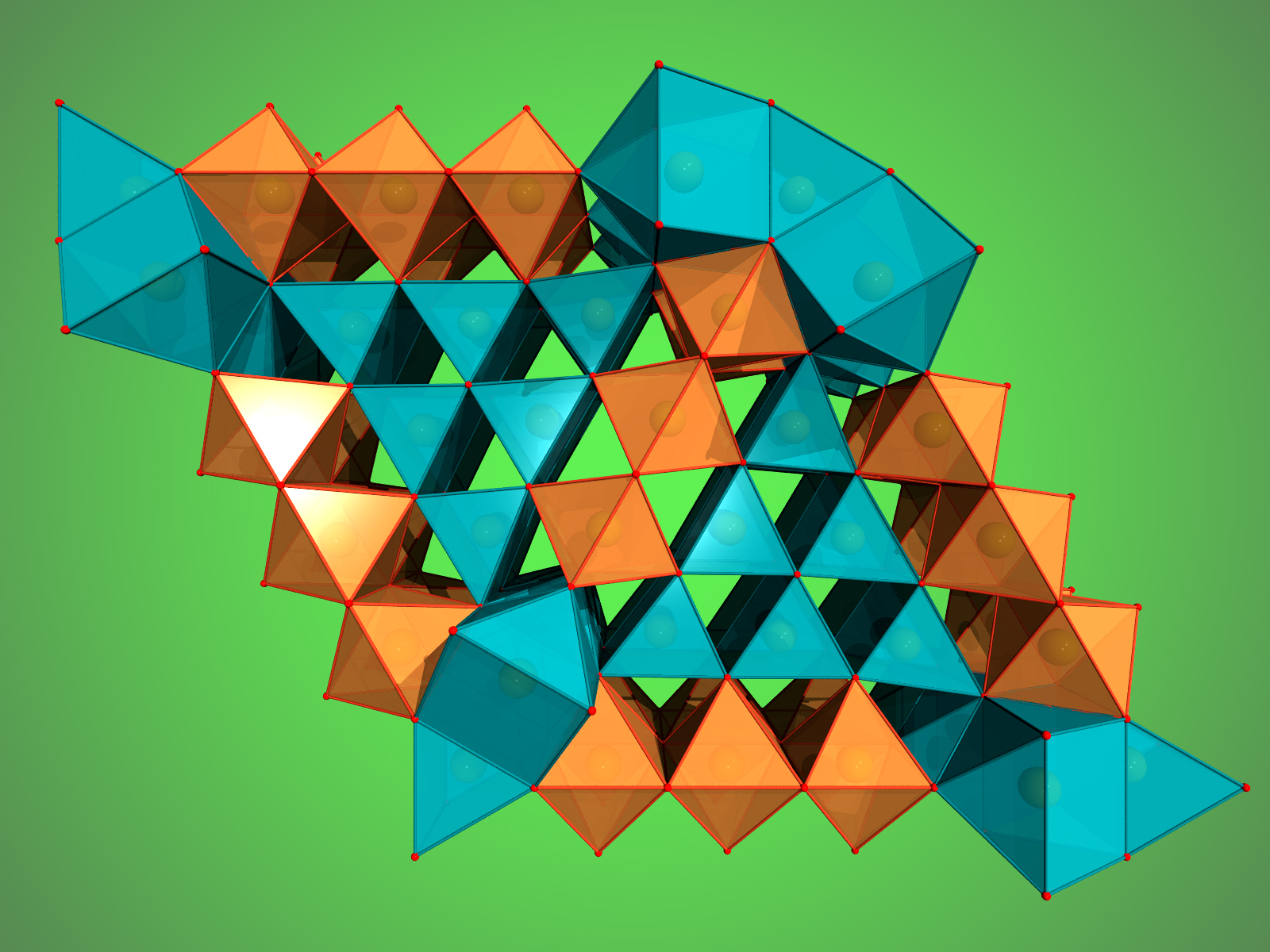
When the scientists applied a pressure of more than 67 GPa (about 670,000 times the standard atmospheric pressure) to their hematite sample and heated it to more than 2400° C, Fe2O3 decomposed and formed Fe5O7 (Fig. 1), an iron oxide that has never been seen. These conditions correspond to roughly 1500 kilometers below the surface of the Earth. At an even higher pressure of 70 GPa corresponding to about 1670 kilometers below the surface, magnetite decomposed and another new iron oxide formed, Fe25O32 (Fig. 2). The formation of both so-far unknown compounds leads to the release of oxygen.
Although iron oxides do not normally exist in the bulk of the Earth’s lower mantle, they can be transported there via subduction zones, where one tectonic plate dives under another. Hematite and magnetite are major components of so-called Banded Iron Formations and ironstones, huge sedimentary rock formations occurring on all continents. These formations may reach up to several hundred meters in thickness and hundreds of kilometers in length. Deposited in the world’s oceans about two billions years ago, Banded Iron Formations form part of the ocean floor and are recycled into the Earth’s interior by subduction to great depths, possibly extending to the core-mantle boundary region.
As the team now observed, at conditions corresponding to the middle of the Earth’s lower mantle hematite and magnetite decompose releasing huge amounts of oxygen-rich fluid (as oxygen is liquid under these conditions). “We estimate that this source so far provided an amount of oxygen equivalent to eight to ten times the mass of oxygen in the atmosphere,” said Bykova. “That's a surprise, and it is not quite clear what happens with the oxygen down there.”
The oxygen-rich fluid could either locally oxidize surrounding materials or pass to the transition zone, or even to the upper mantle. “This remains to be explored,” said co-author Maxim Bykov (University of Bayreuth). “For now, we can only say that there is a huge source of oxygen in the mantle that can significantly affect geochemical processes by changing oxidation states and mobilizing trace elements. This will open a large new field of modelling.”
The discovery of the new iron oxides thus not only adds to the knowledge about fundamental characteristics of these substances, said Bykov. “Our work shows that we maybe miss significant parts of the processes in the Earth. Subducted slabs can apparently produce unexpected things. The effects on Earth's global dynamics, including climate variations, have to be investigated.”
See: E. Bykova1, L. Dubrovinsky1, N. Dubrovinskaia1, M. Bykov1, C. McCammon1, S.V. Ovsyannikov1, H.-P. Liermann2, I. Kupenko1,3, A.I. Chumakov3, R. Rüffer3, M. Hanfland3, and V. Prakapenka4, “Structural complexity of simple Fe2O3 at high pressures and temperatures,” Nat. Commun. 7, 10661 (2016). DOI: 10.1038/ncomms10661
Author affiliations: 1University of Bayreuth, 2Deutsches Elektronen-Synchrotron, 3European Synchrotron Radiation Facility, 4The University of Chicago,
Correspondence: *[email protected]
N.D. and L.D. thank the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) and the Federal Ministry of Education and Research (BMBF, Germany) for funding. N.D. thanks the DFG for financial support through the Heisenberg Program and project no. DU 954-8/1, and BMBF for the grant no. 5K13WC3 (Verbundprojekt O5K2013, Teilprojekt 2, PT-DESY). GeoSoilEnviroCARS is supported by the National Science Foundation-Earth Sciences (EAR-1128799) and Department of Energy-GeoSciences (DE-FG02-94ER14466). This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation’s first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America’s scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.
The U.S. Department of Energy’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.