DNA encodes the genetic information of life and thus plays a major role in biology. But it also moonlights as a mechanical building block in nanotechnology. Researchers have developed ways of using it as a programmable bond to assemble nanoparticle building blocks into a wide-range of crystalline structures. However, these DNA-interconnected nanoparticle superlattices are susceptible to thermal degradation, limiting their use in certain practical applications. One solution is to add molecules that slip—or intercalate—between the DNA base pairs to make the DNA “bonds” stronger. A new study of this intercalation technique used x-rays from the U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne National Laboratory to monitor the structural changes that occur during this process. The researchers identified an intercalator that significantly strengthens DNA bonds and used it to construct a complex, hierarchical superlattice architecture, comprised of gold nanoparticles in its core and quantum dots in its shell, opening possibilities for the fabrication of new, technologically important materials.
DNA-directed nanoparticle assembly is in some ways a materials scientist’s dream. DNA coding allows one to pre-program a set of nanoparticles so that they assemble with nanometer-scale precision into a three-dimensional lattice having practically any desired crystal symmetry.
The first step is to chemically attach DNA strands to the surfaces of a set of nanoparticles. Then, one adds DNA linkers that hybridize (or bond) with the strands anchored to the particles. These linkers have short single-stranded regions—called “sticky ends”—that dangle off the nanoparticles. If their codes (written in the nucleobases) match up, the sticky end of one nanoparticle can bond to the sticky end of another nanoparticle. By carefully arranging these “DNA duplex interconnects,” one can build superlattice architectures with, for example, a body-centered cubic symmetry.
Although this methodology has been utilized to couple nanoparticle building blocks of different compositions together to create complex materials, these architectures can disassemble when heated above the melting temperatures of the DNA interconnects. Researchers have strengthened the bonds by incorporating different types of nucleic acids or changing sequences. However, if the bonds are too strong, the desired versatility and dynamic nature of the assembled structures are reduced. One solution is to build nanostructures using the DNA-directed assembly technique and then post-synthetically add small-molecule intercalators that can bind to the DNA interconnects. These intercalators can insert between the DNA bases without disturbing the original structures, locking them into place at higher temperatures.
In a recent study published in the journal ACS Nano, a research team from Northwestern University investigated a variety of ruthenium-based DNA intercalators (molecules with a ruthenium core, one inserting ligand, and two ancillary ligands). The researchers modified the binding affinities of these molecules to DNA duplexes by altering the sterics (atomic arrangement) and charge of the ancillary ligands.
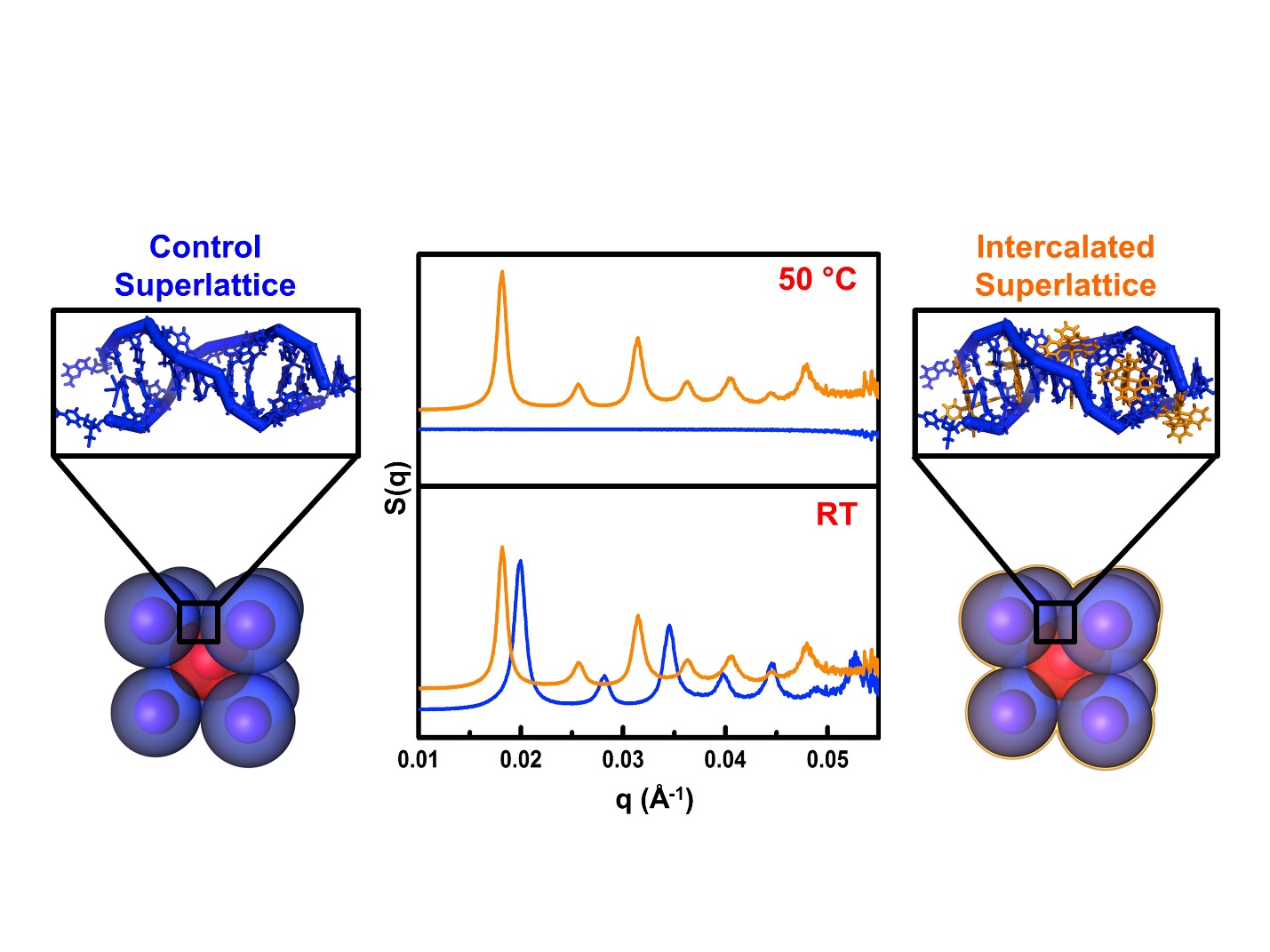
In order to study the effect that the intercalators had on the bond strength, the team incubated the different intercalators in solutions with gold nanoparticle-based superlattices. They then heated each sample and monitored the response of the superlattice structures with small-angle x-ray scattering (SAXS) at the DuPont-Northwestern-Dow Collaborative Access Team 5-ID-B,C,D x-ray beamline at APS. The superlattice structures were detected through specific x-ray diffraction peaks. These peaks disappeared above the melting temperature of the superlattice (Fig. 1).
By comparing the intercalated samples with a control superlattice, the team showed that the intercalators raised the melting temperatures—proof that their presence strengthened the binding of the DNA strands. The intercalator producing the largest effect (called “complex 1”) increased the melting temperature of the superlattices by 15° Celsius above that of the non-intercalated sample. The SAXS data agreed with other measurements of the melting temperature taken with UV-vis spectroscopy.
To demonstrate the power of this technique, the researchers constructed a hierarchical structure made with two types of nanoparticles. They synthesized DNA-nanoparticle superlattices composed of gold nanoparticles and then added complex 1. After the intercalation, the superlattices became “fixed seeds” for further nanoparticle assembly. The team showed this by adding DNA-modified semiconductor quantum dots to their seed solution. The sample was heated and then slow-cooled to allow the DNA-functionalized quantum dots to assemble around the DNA-functionalized gold nanoparticle-based crystal seeds. The seed superlattices withstood the higher temperatures thanks to the intercalators. The resulting core-shell structure, which exhibits unique optical properties, had never been realized before.
The ability to significantly raise the superlattice melting temperature post-synthetically using intercalators opens new possibilities in materials fabrication. — Michael Schirber
See: Soyoung E. Seo, Mary X. Wang, Chad M. Shade, Jessica L. Rouge, Keith A. Brown, and Chad A. Mirkin*, “Modulating the Bond Strength of DNA-Nanoparticle Superlattices,” ACS Nano 10, 1771 (2016). DOI: 10.1021/acsnano.5b07103
Author affiliation: Northwestern University
Correspondence: *[email protected]
This material is based upon work supported by Air Force Office of Scientific Research Award FA9550-11-1-0275, and the Centers of Cancer Nanotechnology Excellence (CCNE) initiative of the National Institutes of Health (NIH) under Award U54 CA151880. This work made use of the EPIC facility (NUANCE Center-Northwestern University), which has received support from the MRSEC program (NSF DMR-1121262) at the Materials Research Center; the International Institute for Nanotechnology (IIN); and the State of Illinois, through the IIN. M. Wang gratefully acknowledges a Graduate Research Fellowship from the National Science Foundation (NSFGRFP) and a Northwestern University Ryan Fellowship. J. Rouge acknowledges a postdoctoral fellowship from the PhRMA foundation. K. Brown gratefully acknowledges support from Northwestern University’s International Institute for Nanotechnology. The DuPont-Northwestern-Dow Collaborative Access Team is supported by Northwestern University, E.I. DuPont de Nemours & Co., and The Dow Chemical Company. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.