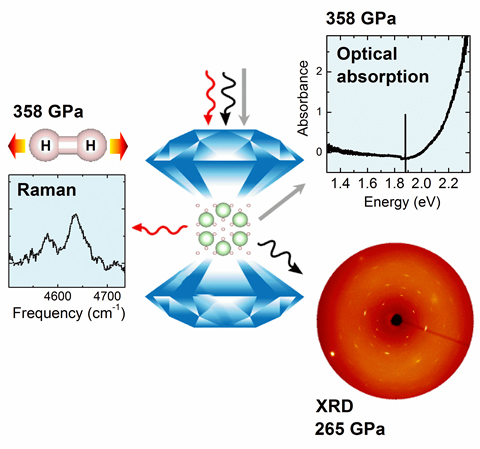
Hydrogen-rich materials have been predicted to be promoters for the metallization of hydrogen. A group of scientists using the U.S. Department of Energy’s Advanced Photon Source (APS) has studied Ar(H2)2, a hydrogen-rich material formed by argon (Ar) and hydrogen (H2), at ultra-high pressures of over three million atmosphere by combining experimental and theoretical methods. Contrary to the previous belief, it was observed that Ar damps the intermolecular interactions between H2 molecules, in effect a “negative” chemical pressure that postpones metallization. The results were published in Proceeding of the National Academic of Sciences, USA.
The hydrogen molecule (H2)is the simplest molecule at ambient conditions. Under extreme compression, intensified intermolecular interactions between H2 molecules transform the originally simple molecules into a different, complex form. For instance, H2 shows an intriguing and exotic structure with proton tunneling at pressures above 230 gigapascals GPa) (2.3 million atmosphere). Further compressing H2 to above 4-million atmosphere pressure was predicted to transform insulating H2 to metallic H2 or even atomic metallic hydrogen, which may be a meta-stable and room-temperature superconductor. However, either probing the exotic structure of the H2 high-pressure phase (H is the weakest scatterer of x-rays) or transforming H2 into a metallic phase presents an extremely grand challenge.
Theories predicted that hydrogen-rich material may help the metallization of hydrogen through chemical pressure from the foreign atoms or molecules. Ar(H2)2 belongs to a typical hydrogen-rich material in the form of the Van der Waals compound. In Ar(H2)2, Ar atoms and H2 molecules are “glued” together by the London dispersion force, so that H2 units are preserved. It provides a unique opportunity to explore its crystal structure (due to the presence of heavier atom Ar), molecular properties (Raman), and electronic properties (optical absorption) at the same time to extremely high pressure.
By applying anx-ray beam with high brilliance and sharp focus at the High Pressure Collaborative Access Team 16-ID-B and GeoSoilEnviroCARS 13-ID-D x-ray beamlines at the APS, x-ray diffraction (XRD) data of Ar(H2)2was able to be measured up to 265 GPa, which is currently a record pressure for studying hydrogen-rich materials by XRD. The molecular vibrational properties and electronic properties were measured by an advanced confocal micro-Raman system with 660-nm excitation to 358 GPa. The APS is an Office of Science user facility at Argonne National Laboratory.
The team’s results suggest that Ar does not facilitate the molecular dissociation and bandgap closure of H2; in fact, it works in the opposite direction (Fig. 1). This work also clarified previous controversies in crystal structures of Ar(H2)2 at very high pressure. The results provide a solid basis for future searches of hydrogen-rich materials that facilitate metallization of hydrogen. — Vitali Prakapenka
See: Cheng Ji, Alexander F. Goncharov, Vivekanand Shukla, Naresh K. Jena, Dmitry Popov, Bing Li, Junyue Wang,Yue Meng, Vitali B. Prakapenka, Jesse S. Smith, Rajeev Ahuja, Wenge Yang, and Ho-kwang Mao*, “Stability of Ar(H2)2 to 358 GPa,” Proc. Nat. Acad. Sci. USA, published ahead of print, March 13, 2017. DOI: 10.1073/pnas.1700049114
Correspondence: *[email protected]
High Pressure Collaborative Access Team (HPCAT) operations are supported by the U.S. Department of Energy (DOE)-National Nuclear Security Administration under Award DENA0001974 and by the DOE-Basic Energy Sciences under Award DE-FG02-99ER45775, with partial instrumentation funding by the National Science Foundation (NSF). A.F.G. and V.B.P. are grateful for NSF Award MRI EAR/IF1531583. GeoSoilEnviroCARS is supported by the NSF—Earth Sciences (Grant EAR-1128799) and DOE—Geosciences (Grant DE-FG02-94ER14466). A.F.G. was partially supported by a Chinese Academy of Sciences Visiting Professorship for Senior International Scientists Grant 2011T2J20 and Recruitment Program of Foreign Experts. This research used resources of the Advanced Light Source, which is a DOE Office of Science User Facility under contract no. DE-AC02-05CH11231. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02- 06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.