High-temperature superconductivity continues to mature as a technology even though the underlying mechanisms responsible for it in cuprates (copper oxides) remain unclear, despite decades of research. Today, the iridium oxide Sr2IrO4 is attracting intensive study because of its many parallels with La2CuO4, the parent compound of some cuprate superconductors. The hope is that when modifications of Sr2IrO4 are discovered that make it superconducting, these will yield insights into the root cause of superconductivity in cuprates, as well. To advance this quest, an international group of researchers carried out spectroscopic x-ray measurements on rhodium-doped Sr2Ir1–xRhxO4 samples at the U.S. Department of Energy’s Advanced Photon Source (APS), an Office of Science user facility at Argonne National Laboratory.
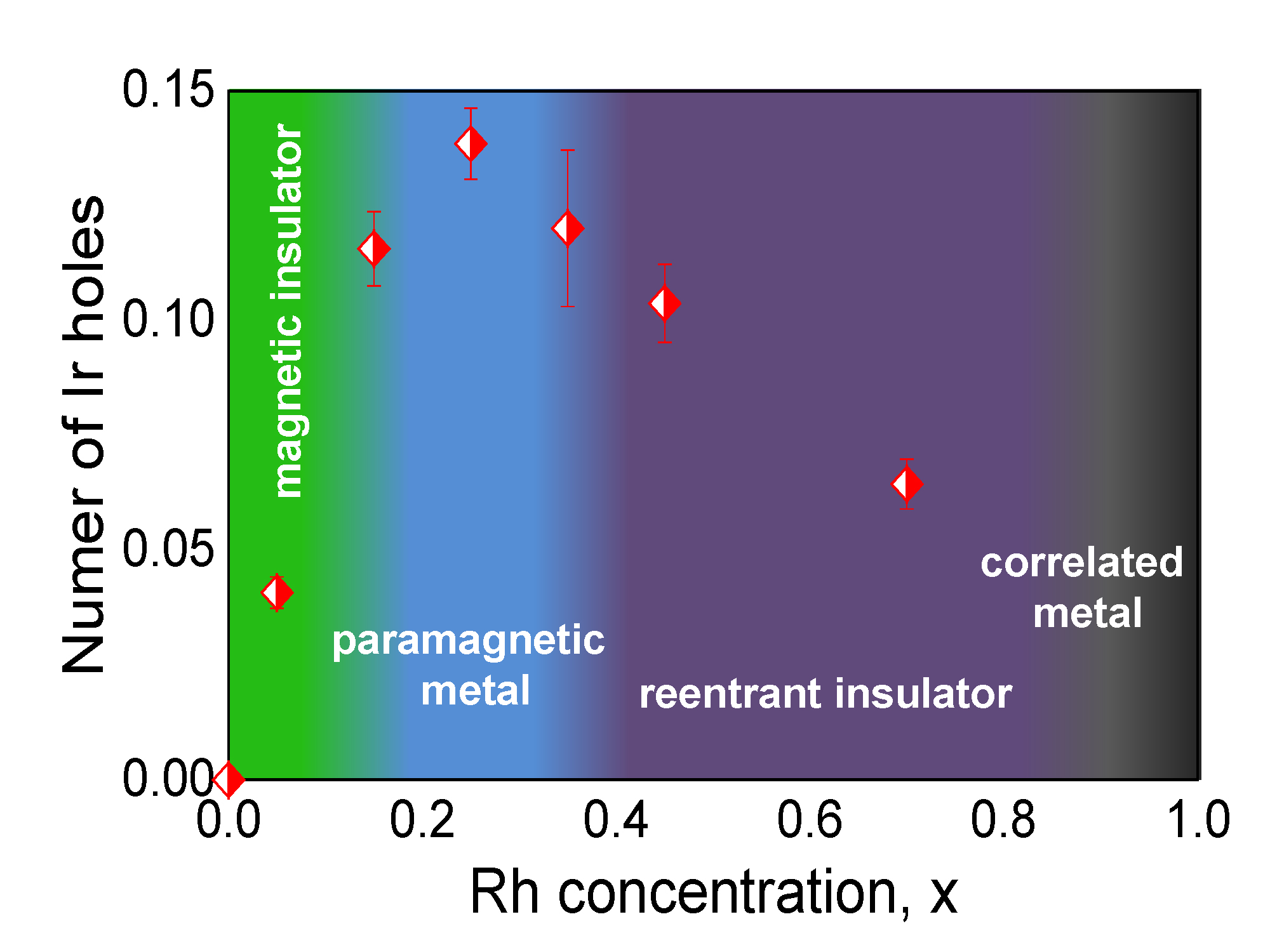
There are theoretical and experimental reasons to believe that superconductivity may be possible in Sr2IrO4 through electron or hole doping, which can be achieved by replacing either strontium (Sr) or iridium (Ir) cations with a non-isovalent ion. One promising approach involves doping with Rh ions to create Sr2Ir1–xRhxO4; however, several fundamental issues regarding the doping process remain to be resolved. Among these are the oxidation state of Rh when it enters the Sr2Ir1–xRhxO4 lattice, the impact of the disparate strength of spin-orbit interactions in 4d (Rh) and 5d (Ir) ions, and how increasing Rh content causes Sr2Ir1–xRhxO4 to switch from an insulator to a metal. Equally mysterious is the reversion of the metallic state to an insulating condition as the Rh doping level continues to increase (Fig.1).
Contrary to earlier reports of Rh being in either an Rh4+ or Rh3+ oxidation state across the compositional range, the researchers in this study, carrying out experiments at the X-ray Science Division 4-ID-D beamline at the Argonne APS, found a smooth evolution from Rh3+ at doping levels below x = 0.05 toward Rh4+ at doping levels above x = 0.70, with the average Rh valence eventually reaching the Rh4+ value in the end compound Sr2RhO4.
At low Rh concentrations, Rh assumes a predominant 3+ state which dopes holes into nearest-neighbor Ir ions, which assume a 5+ oxidation state. This explains the rapid decrease in the system’s resistivity leading to a metallic state at x ~ 0.16, since doped holes at Ir sites reduce the on-site Couloumb repulsion at these sites enabling hopping of charge from site to site (Sr2IrO4 is a Mott insulator). As x continues to increase, and with it the probability of finding Rh-O-Rh bonds, the number of doped holes decreases systematically as the average Rh valence moves toward 4+ (and so does the Ir valence), causing a reentrant insulating phase to emerge at x ~ 0.35. Only a small fraction of Ir ions (less than 25%) assume a 5+ oxidation state across the doping-dependent Rh/Ir charge partitioning.
Although the decrease in the number of charge carriers at high doping level is the most likely explanation for the reentrant insulating phase, the researchers note that doping-induced disorder is also likely to be at play. These findings should prove key to informing ongoing searches for novel electronic phases, including superconductivity, in (5d) Iridium-oxide compounds doped with 4d (or 3d) elements where charge disproportionation is expected. — Vic Comello
See: S. Chikara,1,2 G. Fabbris,1,3,4 J. Terzic,5 G. Cao,5 D. Khomskii,6 and D. Haskel,1,* “Charge partitioning and anomalous hole doping in Rh-doped Sr2IrO4,” Phys. Rev. B 95, 060407 (Rapid Communications) (2017); manuscript selected by PRB editors as an Editors’ Suggestion.
Author affiliations: 1Argonne National Laboratory, 2Los Alamos National Laboratory, 3Washington University, 4Brookhaven National Laboratory, 5University of Colorado-Boulder, 6Universität zu Köln
Correspondence: *[email protected]
The work at the Advanced Photon Source, Argonne National Laboratory, is supported by the U.S. Department of Energy (DOE) Office of Science under Grant No. DEAC02-06CH11357. Work at Los Alamos National Laboratory is supported by the Laboratory-Directed Research and Development program. The NHMFL Pulsed-Field Facility is funded by the U.S. National Science Foundation through cooperative Grant No. DMR-1157490, the State of Florida, and the DOE. Work at Brookhaven National Laboratory is supported by the DOE Office of Basic Sciences under Contract No. DE-SC00112704 and the Early Career Award Program under Award Number 1047478. G.C. acknowledges support of U.S. National Science Foundation grants DMR-1265162 and DMR-1712101. The work of D.Kh. was supported by DFG via the project SFB 1238 and by Köln University via the German Excellence Initiative.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.