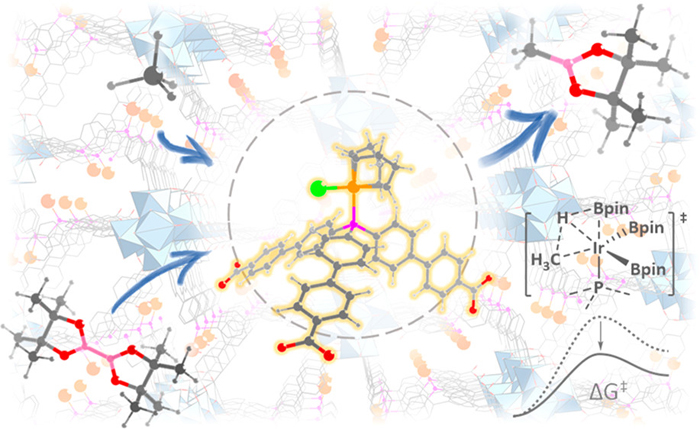
 Just because a molecule is small and simple does not make it easy to manipulate. Methane, a single carbon bonded to four hydrogen atoms, is the simplest hydrocarbon molecule. Due to its abundance in shale gas and other sources, methane has extraordinary potential as a building block for a wide range of useful chemicals, from fuels to polymers. However, converting methane to these chemicals is difficult because of chemistry challenges, limiting methane’s potential. In this work, researchers describe a novel approach to methane activation: borylation with catalytic help from a metal-organic framework (MOF). Using the U.S. Department of Energy's Advanced Photon Source (APS) at Argonne National Laboratory, the team characterized the MOF and demonstrated how to jumpstart methane borylation, offering a promising strategy for getting more out of methane.
Just because a molecule is small and simple does not make it easy to manipulate. Methane, a single carbon bonded to four hydrogen atoms, is the simplest hydrocarbon molecule. Due to its abundance in shale gas and other sources, methane has extraordinary potential as a building block for a wide range of useful chemicals, from fuels to polymers. However, converting methane to these chemicals is difficult because of chemistry challenges, limiting methane’s potential. In this work, researchers describe a novel approach to methane activation: borylation with catalytic help from a metal-organic framework (MOF). Using the U.S. Department of Energy's Advanced Photon Source (APS) at Argonne National Laboratory, the team characterized the MOF and demonstrated how to jumpstart methane borylation, offering a promising strategy for getting more out of methane.
Methane’s chemical stubbornness has two main causes. First, methane’s carbon-hydrogen bonds are extremely strong, making the molecule resistant to chemical reactions. Second, selectivity issues can lead to the production of unwanted side products. Lengthy and energy-intensive high-temperature reaction conditions can overcome these challenges, but chemists for obvious reasons would prefer a simpler way to activate methane under mild conditions.
Borylation is a common strategy for activating otherwise inert molecules, such as methane. The reaction creates an easily cleavable carbon-boron bond that is primed for downstream reactions. Yet, previous attempts to borylate methane have met with mixed results, often resulting in products with multiple boron atoms, which is problematic for downstream reactions. Moderate progress has come from homogenous reaction conditions using phenanthroline- and diphosphine-iridium complexes to promote methane borylation under mild conditions, but catalytic activity and selectivity remain less than ideal.
In this study, the research team turned toward MOFs to solve their catalytic and selectivity challenges. MOFs are a class of compounds that include a metal or metal cluster, in this case zirconium, coordinated by organic ligands to form, again in this case, a three-dimensional structure. The coordinated iridium serves as the catalytic center, while the surrounding framework can be carefully selected and formed to optimize a reaction. Rather than homogenous conditions, MOFs can, by virtue of their rigid structure, offer greater control over a chemical reaction by exerting the spatial arrangement of atoms and directional control over the reaction.
The researchers studied a series of mono(phosphine)-Ir-based MOFs, and found one, Zr-P1-Ir, that outperformed other complexes for catalyzing the mono-borylation of methane, providing an impressive turnover number of 127 at a relatively mild temperature of 110° C. To better understand the mechanism of the highly performing MOF, which may help the researchers improve performance further, the researchers collected x-ray absorption spectra of the MOFs at the Materials Research Collaborative Access Team 10-BM-B x-ray beamline of the APS. (The APS is an Office of Science user facility.) The data revealed that Zr-P1-IR adopts a distorted tetrahedral geometry with one chloride, one bidentate COD (1,5-cyclooctadiene), and one mono(phosphosphine) from the MOF ligand.
Additional x-ray absorption near edge structure data, also collected at the MR-CAT beamline, uncovered the metal center oxidation states. This structural data, combined with density functional theory calculations, suggested that MOFs stabilize highly active and low-coordinate Ir complexes that are key for promoting catalytic methane borylation. The authors say that this strategy may be generally applied, beyond methane, for the activation of inert compounds. — Erika Gebel Berg
See: Xuanyu Feng1, Yang Song1, Zhe Li1,2, Michael Kaufmann1, Yunhong Pi1,3, Justin S. Chen1, Ziwan Xu1, Zhong Li3, Cheng Wang2, and Wenbin Lin1*, “Metal−Organic Framework Stabilizes a Low-Coordinate Iridium Complex for Catalytic Methane Borylation,” J. Am. Chem. Soc. 141(28), 11196 (2019). DOI: 10.1021/jacs.9b04285
Author affiliations: 1The University of Chicago, 2State Key Laboratory of Physical Chemistry of Solid Surface, 3South China University of Technology
Correspondence: * [email protected]
This work was supported by the National Science Foundation (Grant CHE-1464941). Z. Li and Y. Pi acknowledge financial support from the China Scholarship Council. We thank Mr. Wenbo Han and Mr. Taokun Luo for experimental help. MR-CAT operations are supported by the U.S. Department of Energy (DOE) and the MR-CAT member institutions. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
