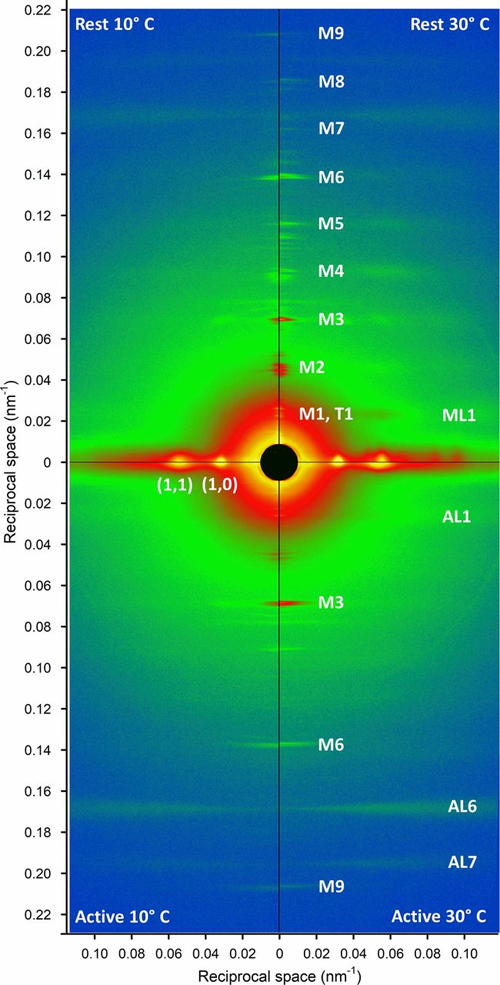
Striated muscle contraction is a highly regulated process that involves an orchestrated series of events within the muscle’s contractile units, which are also known as sarcomeres. In a recent study, which involved collecting x-ray diffraction data at the U.S. Department of Energy’s (DOE’s) Advanced Photon Source (APS), researchers studied the effect of low temperature on mammalian skeletal muscle contraction. They found that cooler temperatures reduce force generation by trapping filaments in the muscle sarcomeres in a refractory state that cannot undergo contraction and utilize adenosine triphosphate (ATP). This mechanism provides important insight into how hibernating animals may conserve energy while still allowing vital functions in the body to continue.
Muscle tissue’s repeating functional units, the sarcomeres, impart a striated or striped appearance to the tissue when examined under a microscope. Sarcomeres contain thin (containing the protein actin) and thick (containing the protein myosin) filaments that power contraction of skeletal muscle and cardiac muscle, both of which are striated.
Contraction of striated muscle is triggered when calcium enters the muscle fibers. This leads to structural changes in proteins known as troponin and tropomyosin that reside in the thin filament. Ultimately, this allows actin and myosin to interact with each other in a way that allows hydrolysis of ATP, the source of energy for muscle contraction.
However, recent studies have shown that structural changes in the thick filament play an important regulatory role in contraction. Researchers have discovered that when striated muscle is in its relaxed state, myosin is trapped in helical tracks on the surface of the thick filament, thus preventing its interaction with actin and hydrolysis of ATP. Additional studies have also found that myosin protein in relaxed muscle is sensitive to temperature changes.
A multi-national, multi-institution group of researchers recently set out to investigate the structural changes that take place in the thick filament of mammalian skeletal muscle in response to temperature changes. They vertically mounted sections of mouse skeletal muscle on the x-ray path of the Biophysics Collaborative Access Team (Bio-CAT) 18-ID-D x-ray beamline at the APS (the APS is an Office of Science user facility at Argonne National Laboratory). Then, they collected x-ray diffraction patterns (Fig. 1) from the muscle at different temperatures ranging from near-physiological at 35° C to 10° C.
Their results showed that lowering the temperature below the physiological level converts the thick filament from its ordered OFF state to a disordered one in which myosin is trapped in a refractory state that cannot bind actin or utilize ATP. Also, this effect is temperature-dependent. Progressive reductions in temperature lead to more myosins being disrupted on the surface of the thick filament. Indeed, only half as many thick filaments were left in the ordered OFF state at 10° C as were present at 35° C.
The drop from physiological temperature to 10° C also produced a 3-fold reduction in the amount of force generated by the mouse muscle. This refractory state of the thick filament may help explain the decreased power output of mammalian muscle at cooler temperatures, and this could have important implications for hibernating animals. When animals enter hibernation, their body temperature drops to near ambient level, and their energy-costly metabolic activities also decrease. Based on the results of this study, which show that ATP hydrolysis and energy output also drop substantially in resting muscle under cooler temperatures, the researchers suggest that this mechanism may contribute to helping animals conserve energy during hibernation. Because muscles comprise approximately 40% of mammalian body mass, having a regulatory mechanism like this at low temperatures can further reduce ATP consumption during hibernation, while still maintaining the body’s vital functions. ― Nicola Parry
See: Marco Caremani1, Elisabetta Brunello1‡, Marco Linari1,2, Luca Fusi3, Thomas C. Irving4, David Gore4, Gabriella Piazzesi1, Malcolm Irving3, Vincenzo Lombardi1*, and Massimo Reconditi1,2, “Low temperature traps myosin motors of mammalian muscle in a refractory state that prevents activation,” J. Gen. Physiol. 151(11), 1272 (2019). DOI: 10.1085/jgp.201912424
Author affiliations: 1University of Florence, 2Consorzio Nazionale Interuniversitario per le Scienze Fisiche della Materia, 3King’s College London, 4Illinois Institute of Technology ‡Present address: King’s College London
Correspondence: * [email protected]
This project was supported by Fondo per gli Investimenti della Ricerca di Base (Futuro in Ricerca project RBFR08JAMZ; Italy), Consorzio Nazionale Interuniversitario per le Scienze Fisiche della Materia (Progetto d’Innesco della Ricerca Esplorativa 2007; Italy), and Ente Cassa di Risparmio di Firenze (2012.0611; Italy); Medical Research Council (MR/M026655/1; U.K.); and the National Institute of General Medical Sciences of the National Institutes of Health grant P41 GM103622 to Bio-CAT. This research used resources of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357, and the Canadian Light Source and its funding partners.
Bear photo: MJ Boswell from Annapolis., MD
https://commons.wikimedia.org/wiki/File:Bear_Sleeping_%2811842384304%29.jpg
The U.S. Department of Energy’s Advanced Photon Source at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
