The original Brookhaven National Laboratory news release can be read here.
Because platinum is rare and expensive, scientists have been seeking ways to create catalysts that use less of this precious metal. Understanding exactly what the platinum does is an essential step. Using a number of U.S. Department of Energy (DOE)-funded experimental resources, including experiment facilities at Brookhaven National Laboratory and high-brightness x-rays from the DOE’s Advanced Photon Source (APS), scientists from Stony Brook University (SBU), Brookhaven, and other collaborating institutions have uncovered dynamic, atomic-level details of how an important platinum-based catalyst works in the water gas shift reaction. This reaction transforms carbon monoxide (CO) and water (H2O) into carbon dioxide (CO2) and hydrogen gas (H2), an important step in producing and purifying hydrogen for multiple applications, including use as a clean fuel in fuel-cell vehicles, and in the production of hydrocarbons.
The new study, published in the journal Nature Communications, identifies the atoms involved in the catalyst's active site, resolving earlier conflicting reports about how the catalyst operates. The experiments provide definitive evidence that only certain platinum atoms play an important role in the chemical conversion.
"Part of the challenge is that the catalyst itself has a complex structure," explained lead author Yuanyuan Li, a research scientist at SBU's Materials Science and Chemical Engineering Department who has a guest appointment in Brookhaven Lab's Chemistry Division and works under the guidance of Brookhaven/SBU joint appointee Anatoly Frenkel.
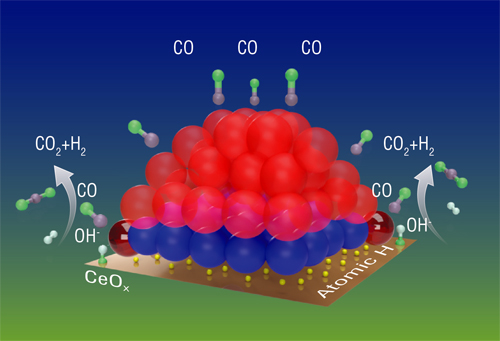
"The catalyst is made of platinum nanoparticles (clumps of platinum atoms) sitting on a cerium oxide (ceria) surface. Some of those platinum atoms are on the surface of the nanoparticle, some are in the core; some are at the interface with ceria, and some of those are at the perimeter--the outside edges--of that interface," Li said. "Those positions and how you put the particles on the surface may influence which atoms will interact with the support or with gas molecules, because some are exposed and some are not."
Earlier experiments had produced conflicting results about whether the reactions occur on the nanoparticles or at single isolated platinum atoms, and whether the active sites are positively or negatively charged or neutral. Details of how the ceria support interacts with the platinum to activate it for catalytic activity were also unclear.
"We wanted to address these questions," said Li. "To identify the active site and determine what is really happening at this site, it is better if we can investigate this type of catalyst at the atomic level," she noted.
The team, which included scientists from Brookhaven's Center for Functional Nanomaterials (CFN) and other institutions throughout the U.S. and in Sweden, used a range of techniques to do just that. They studied the catalyst under reaction conditions and, unexpectedly, captured a peculiar effect that occurred when the catalysts reached their active state in reaction conditions.
"The platinum atoms at the perimeter of the particles were 'dancing' in and out of focus in an electron microscopy experiment carried out by our collaborators, while the rest of the atoms were much more stable," Frenkel said. Such dynamic behavior was not observed when some of the reactants (CO or water) were removed from the stream of reacting molecules.
"We found that only the platinum atoms at the perimeter of the interface between the nanoparticles and ceria support provide the catalytic activity," Li said. "The dynamic properties at these perimeter sites allow the CO to get oxygen from the water so it can become CO2, and the water (H2O) loses oxygen to become hydrogen."
Now that the scientists know which platinum atoms play an active role in the catalyst, they may be able to design catalysts that contain only those active platinum atoms.
"We might assume that all the surface platinum atoms are working, but they are not," Li said. "We don't need them all, just the active ones. This could help us make the catalyst less expensive by removing the atoms that are not involved in the reaction. We believe that this mechanism can be generalized to other catalytic systems and reactions," she added.
Electron microscopy "snapshots" at the CFN and at the National Institute of Standards and Technology revealed the dynamic nature of the perimeter platinum atoms. "In some images, the perimeter site is there, you can see it, but in some images, it is not there. This is evidence that these atoms are very dynamic, with high mobility," Li said.
Infrared (IR) spectroscopy studies in Brookhaven's Chemistry Division revealed that the appearance of the perimeter sites coincided with "oxygen vacancies"—a kind of defect in the cerium oxide surface. These studies also showed that CO tended to migrate across the platinum nanoparticle surface toward the perimeter atoms, and that hydroxy (OH) groups lingered on the ceria support near the perimeter platinum atoms.
"So it seems like the perimeter platinum atoms bring the two reactants, CO and OH (from the water molecules) together," Li said.
X-ray photoelectron spectroscopy studies in the Chemistry Division revealed that perimeter platinum atoms also became activated—changed from a nonmetallic to a metallic state that could capture oxygen atoms from the OH groups and deliver that oxygen to CO. "This really shows that these activated perimeter platinum sites enable the reaction to take place," Li said.
A final set of experiments—x-ray absorption spectroscopy studies conducted at the X-ray Science Division Spectroscopy Group’s 20-ID-C x-ray beamline at the APS, an Office of Science user facility at the DOE’s Argonne National Laboratory—showed the dynamic structural changes of the catalyst.
"We see the structure is changing under reaction conditions," Li said.
Those studies also revealed an unusually long bond between the platinum atoms and the oxygen on the ceria support, suggesting that something invisible to the x-rays was occupying space between the two.
"We think there is some atomic hydrogen between the nanoparticle and the support. X-rays can't see light atoms like hydrogen. Under reaction conditions, those atomic hydrogens will recombine to form H2," she added.
The structural features and details of how the dynamic changes are connected to reactivity will help the scientists understand the working mechanism of this particular catalyst and potentially design ones with better activity at lower cost. The same techniques can also be applied to studies of other catalysts.
See: Yuanyuan Li 1*, Matthew Kottwitz2, Joshua L. Vincent3, Michael J. Enright2, Zongyuan Liu4, Lihua Zhang4, Jiahao Huang1, Sanjaya D. Senanayake4, Wei-Chang D. Yang5, 6, Peter A. Crozier3, Ralph G. Nuzzo2, 7, and Anatoly I. Frenkel1, 4, “Dynamic structure of active sites in ceria supported Pt catalysts for the water gas shift reaction,” Nat. Commun. 12, 914 (2021). DOI: 10.1038/s41467-021-21132-4
Author affiliations: 1Stony Brook University, 2University of Illinois Urbana-Champaign, 3Arizona State University, 4Brookhaven National Laboratory, 5National Institute of Standards and Technology, 6University of Maryland, 7KTH Royal Institute of Technology
Correspondence: * [email protected]
Y.L., A.I.F., and R.G.N. acknowledge support for this work by the U.S. DOE Office of Science-Basic Energy Sciences under Grant No. DE-FG02-03ER15476. Reaction tests and DRIFTS measurements at Brookhaven National Laboratory’s Chemistry Division and XAFS data analysis were made possible due to the Program Development fund 21-017 to A.I.F. M.K. and R.G.N. acknowledge fellowship support from the Department of Chemistry at the University of Illinois. Work by S.D.S. and Z.L. at Brookhaven National Laboratory was supported by the U.S. DOE Office of Science-Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences under contract no. DE-SC0012704. S.D.S. is partially supported by a U.S. DOE Early Career Award. This research used Hitachi2700C STEM of the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. J.L.V. and P.A.C. gratefully acknowledge National Science Foundation grant CBET- 1604971 and the facilities at the National Institute of Standards and Technology at Gaithersburg, M.D., as well as those at the John M. Cowley Center for High Resolution Electron Microscopy at Arizona State University. W.D.Y. acknowledges support under the Cooperative Research Agreement between the University of Maryland and the National Institute of Standards and Technology Physical Measurement Laboratory, award 70NANB14H209, through the University of Maryland. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
