Fuel cells hold tremendous appeal in the energy sector, especially for transportation applications, as they offer the possibility of providing pollution-free power. However, achieving efficient cost-effective fuel cells, especially for the automotive industry, remains a challenge, with one key obstacle being the costly platinum-containing catalysts. Lower-cost platinum-group, metal-free catalysts, such as those based on cobalt (Co), are under investigation, but have exhibited lower activity and durability than those based on platinum. To improve performance, researchers studied the atomic structure of a Co-based catalyst using the U.S. Department of Energy’s (DOE’s) Advanced Photon Source (APS). These studies were also correlated with results from scanning transmission electron microscopy coupled with electron energy loss characterization performed at the DOE’s Oak Ridge National Laboratory. Together, the findings from these studies, along with fuel cell performance and durability data, supports Co-based catalysts as potential game-changers in the race to establish fuel cells as a sustainable energy solution.
Proton exchange membrane fuel cells (PEMFCs) are efficient and clean electricity-generating devices. They generate electricity through the oxidation of hydrogen on an anode catalyst and reduction of oxygen in air on the cathode catalyst, separated by a polymer electrolyte that, as the name suggests, forms a proton-conducting membrane. One of the main advantages of these cells, especially for automotive power, is they can work at ambient temperatures, and they can continuously provide power from hydrogen fuel stored on-board. However, the performance of PEMFCs is highly dependent on the activity of their oxygen-reducing catalyst. This typically includes a platinum-group metals, which are expensive, driving up the cost of the fuel cell. Scientists are exploring transition metal (M) and nitrogen (N) co-doped carbon (C) catalysts (M-N-C catalysts) as an alternative. Iron (Fe) is a promising metal, offering high oxygen reduction efficiency, but Fe-N-C catalysts are prone to degradation in the acidic fuel cell environment, leading to a reduction in efficiency. An even bigger issue with Fe-N-C catalysts is side reactions, called “Fenton reactions,” that produce radicals that degrade the membrane and the catalyst itself.
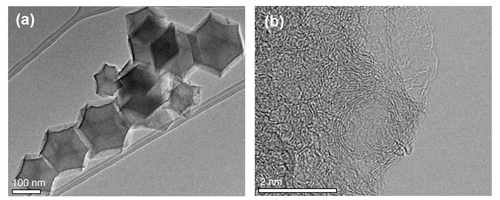
To develop robust catalysts, scientists are investigating Co-N-C catalysts, which is less active toward Fenton reactions and, in general, show better durability than Fe-N-C catalysts. However, cobalt-based catalysts have yet to achieve the oxygen reduction activity of iron, let alone platinum, catalysts. But there are ways to improve the activity of Co-N-C catalysts by, for example, increasing the density of cobalt active sites in the catalyst or optimizing the geometry of the cobalt within the catalyst. In this study, the researchers synthesized a Co-N-C catalyst with a high cobalt content, to increase active site density, by immobilizing the cobalt in a zeolite imidazole framework. These frameworks include micropores that become anchors for the CoN moieties.
After creating these novel catalysts, the researchers from universities and DOE national laboratories tested their catalytic activity in a PEMFC, finding higher activity than that of non-iron platinum-group-metal-free catalysts in the literature. They achieved a current density, 0.022 A cm−2 at 0.9 ViR-free and 0.044 A cm−2 at 0.87 ViR-free (only 30-mV less than the target given by the U.S. DOE for fuel cells). They also tested durability, finding a 4-fold enhanced durability relative to similarly synthesized Fe-N-C catalysts, potentially due to a reduction in Fenton reactions. They also noted that the cobalt catalysts retained their metal centers better than their iron-based counterparts, with a 5-fold reduction in demetallation.
Extensive physical characterization was carried out using Co K-edge x-ray absorption spectroscopy experiments at the Materials Research Collaborative Access Team (MR-CAT) beamline 10-ID-B at the Advanced Photon Source at the DOE's Argonne National Laboratory among other strategies, including x-ray photoelectron spectroscopy using the Environmental Molecular Sciences Laboratory (EMSL) at the DOE’s Pacific Northwest National Laboratory, scanning tunneling electron microscopy (STEM) at the DOE’s Oak Ridge National Laboratory, and electron microscopy at the DOE’s Argonne Center for Nanophase Materials Sciences. These studies showed that the cobalt sites within the zeolite-derived nitrogen-doped carbon substrate were well dispersed (i.e., not clustered). This is key, the researchers say, because previous attempts to increase cobalt density has led to the formation of cobalt nanoparticles, which have low catalytic activity compared to that of atomically-dispersed Co sites. The rigid porphyrin-like structure that houses the cobalt in the zeolite micropores facilitates the atomic dispersion of Co during the formation of the catalyst from these zeolite precursors. While automotive PEMFCs have been commercialized, they are costly due in large part to the high loadings of costly platinum. The development of promising robust and active cobalt catalysts in this work is a first step in replacing platinum in PEMFCs and addressing one of the main challenges to the widespread adoption of this efficient and clean power source. ― Erika Gebel Berg
See: Xiaohong Xie1, Cheng He2, Boyang Li3, Yanghua He4, David A. Cullen5, Evan C. Wegener6, A. Jeremy Kropf6, Ulises Martinez7, Yingwen Cheng8, Mark H. Engelhard1, Mark E. Bowden1, Miao Song1, Teresa Lemmon1, Xiaohong S. Li1, Zimin Nie1, Jian Liu1, Deborah J. Myers6, Piotr Zelenay7, Guofeng Wang3, Gang Wu4*, Vijay Ramani2**, and Yuyan Shao1***, “Performance enhancement and degradation mechanism identification of a single-atom Co–N–C catalyst for proton exchange membrane fuel cells,” Nat. Catal. 3, 1044 (December 2020). DOI: /10.1038/s41929-020-00546-1
Author affiliations: 1Pacific Northwest National Laboratory, 2Washington University in St Louis, 3University of Pittsburgh, 4The State University of New York (University at Buffalo), 5Oak Ridge National Laboratory, 6Argonne National Laboratory, 7Los Alamos National Laboratory, 8Northern Illinois University
Correspondence: * [email protected], ** [email protected], *** [email protected]
The authors acknowledge support from the U.S. Department of Energy (DOE) Energy Efficiency and Renewable Energy, Hydrogen and Fuel Cell Technologies Office (DOE-EERE-HFTO) through the Electrocatalysis consortium (ElectroCat) and the DOE program managers, D. Papageorgopoulos, S. Thompson, D. Peterson and G. Kleen. The EMSL(grid.436923.9) is a U.S. DOE Office of Science user facility sponsored by the Biological and Environmental Research program. Pacific Northwest National Laboratory is operated by Battelle for the U.S. DOE under contract DE-AC05- 76RLO1830. MRCAT at the Advanced Photon Source (APS), a DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02- 06CH11357. The Materials Research Collaborative Access Team is supported both by the DOE and the MR-CAT member institutions. Use of the Center for Nanoscale Materials and Advanced Photon Source, both Office of Science user facilities, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
