Superconductors are mysterious. By some not fully understood mechanism, electricity flows through superconductors with zero resistance. This phenomenon drives the powerful magnets that underpin magnetic resonance imaging and particle accelerators. Although the temperatures needed for superconductivity are typically near absolute zero, limiting the practicality and application of this technology, there are classes of copper-based high-temperature superconductors that have been eyed as platforms for superconducting technologies. Recently, researchers have discovered that nickel, a neighbor of copper on the periodic table, can also superconduct as an oxide, but so far only as an atomically thin film. In a search for superconductivity in more three-dimensional bulk nickel oxides, researchers from Argonne’s Materials Science Division explored this material’s structure, electronics, and quantum behavior using data collected at the U.S. Department of Energy’s Advanced Photon Source (APS). Their findings, published in the journal Chemistry of Materials, may help explain why these crystals do not superconduct and how they might be coaxed into this exotic quantum state.
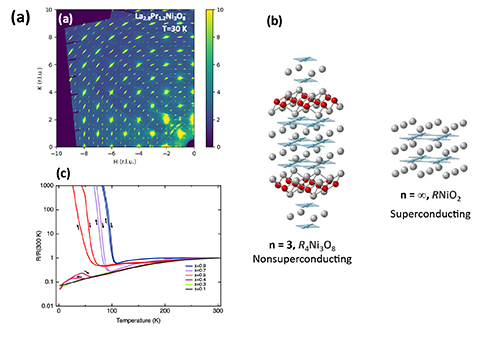
In the current work, researchers studied a non-superconductive trilayer nickelate, R4Ni3O8, which shares many of the “ingredients” for superconductivity with copper-based high-temperature superconductors. The R in R4Ni3O8 stands for “rare earth,” a group of elements at the bottom of the Periodic Table that includes lanthanum (La) and praseodymium (Pr). What inspired researchers to do the current experiment was a curious observation that, in the ground state, nickelate crystals with R=Pr are metallic while those with R=La exhibit an insulating charge- and spin-stripe order. The latter is an unusual state in which electrons “line up” in rows and stand still rather than moving around freely. Somewhere between Pr4Ni3O8 and La4Ni3O8 the metallic electrons must become stripy and vice-versa. Such a T=0 transition is called a quantum phase transition, made possible by the same phenomena that underlie that Heisenberg Uncertainty principle. Quantum phase transitions are predicted in cuprate superconductors too, meaning that details of such a transition in the nickelate may shed light on the secrets of cuprates. Ironically, however, the fact that R4Ni3O8 does not superconduct makes it easier to study this quantum phase behavior, unobscured by superconductivity.
To better understand this potential quantum phase transition between the ground states, the researchers grew a series of crystals with the formula (Pr1-xLax)Ni3O8, (x=0.1, 0.3, 0.4, 0.45, 0.5, 0.7, and 0.9). They performed powder x-ray diffraction at the APS X-ray Science Division (XSD) Structural Science Group’s x-ray beamline 11-BM-B, while collecting single-crystal x-ray diffraction data at the XSD Magnetic Materials Group’s beamline 6-ID-D (Fig. 1), both at APS, an Office of Science user facility at Argonne National Laboratory. These x-ray experiments most directly probe the existence and behavior of the charge stripes. The researchers also measured the crystals' heat capacity, magnetic susceptibility, and electric resistivity, among other characteristics.
Together, these magnetic, transport, thermodynamic, and scattering measurements helped the researchers construct an electronic and magnetic phase diagram of the transition from the metallic to the spin-stripe order states. They also found evidence that there is indeed a T = 0 transition occurring when x = 0.45, give or take. The authors propose two possible models for (Pr1-xLax)Ni3O8 that are consistent with the observed x dependence of its competing phases: an electronically inhomogenous system or a homogenous system. Both scenarios offer interesting science, with the former serving as a way to explore how disorder impacts quantum transitions, and the latter potentially providing a clean quantum critical point at the phase boundary. The evidence seems to weigh in favor of the inhomogeneous scenario, but the jury is still out.
Many scientists believe that we have only scratched the surface of what is possible with superconductivity. Overcoming temperature limitations won't be easy, but basic research into the fundamental physics behind high-temperature superconductivity―like that described in this study―puts scientists on the path to make a quantum leap in superconductor technology. ― Erika Gebel Berg
See: Xinglong Chen*, Hong Zheng, Daniel P. Phelan, Hao Zheng, Saul H. Lapidus, Matthew J. Krogstad, Raymond Osborn, Stephan Rosenkranz, and J. F. Mitchell**, “Competing Charge/Spin-Stripe and Correlated Metal Phases in Trilayer Nickelates (Pr1−xLax)4Ni3O8,” Chem. Mater. 34, 4560 (2022). DOI: 10.1021/acs.chemmater.2c00371
Author affiliation: Argonne National Laboratory
Correspondence: * [email protected], ** [email protected]
This work was supported by the U.S. Department of Energy (DOE) Office of Science-Basic Energy Sciences, Materials Science and Engineering Division. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
